Heart rate variability (HRV) biofeedback training improved myocardial blood flow (MBF) in patients with coronary heart disease (CHD), according to a new study published in JAMA Network Open.1
HRV biofeedback, a stress-reduction intervention, can…
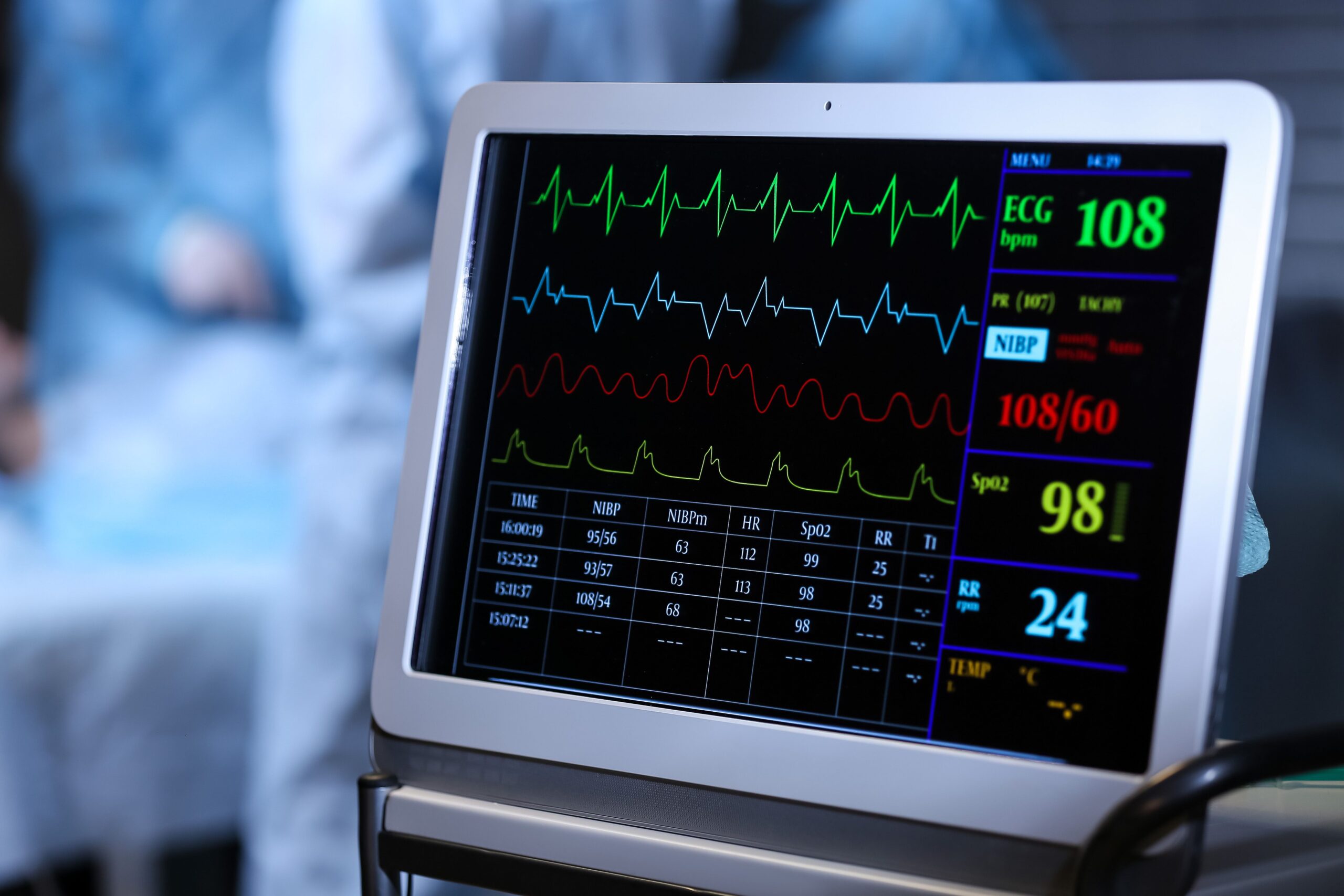
Heart rate variability (HRV) biofeedback training improved myocardial blood flow (MBF) in patients with coronary heart disease (CHD), according to a new study published in JAMA Network Open.1
HRV biofeedback, a stress-reduction intervention, can…

Fewer cycles of platinum-based chemotherapy improved patient-reported outcomes (PROs) vs a longer duration of therapy prior to maintenance avelumab (Bavencio) without compromising treatment efficacy in patients with advanced urothelial cancer, according to findings from the phase 2 DISCUS trial (NCT06892860) presented during the
Data from the study demonstrated that patients who received 3 cycles of chemotherapy (n = 98) experienced a mean change in The European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (EORTC QLQ-C30) global health status/quality of life (QOL) score of 0.0 (95% CI, –5.0-5.2) compared with –8.5 (95% CI, –14.1 to –2.9) among those who received 6 cycles (n = 104). The difference of 8.5 (95% CI, 0.7-16.3; P = .016) significantly favored the 3-cycle arm, meeting the primary end point of the study. Notably, no difference for time to deterioration in global health status/QOL score was observed between groups based on EORTC QLQ-C30 outcomes (HR 0.81, 95% CI 0.46-1.43).
“More patients [who received] 3 cycles of platinum-based chemotherapy went on to receive maintenance avelumab, which may facilitate long-term efficacy,” Enrique Grande, MD, the director of the Department of Medical Oncology at Quirónsalud in Madrid, Spain, and an adjunct professor at the University of Texas MD Anderson Cancer Center in Houston, said during the presentation. “The most important highlight coming from the DISCUS trial is that we can explore the need for [fewer] cycles of combination [chemotherapy] in the era of antibody-drug conjugates in metastatic urothelial cancer.”
DISCUS was an adaptive, open-label study that enrolled patients with locally advanced or metastatic urothelial cancer who were eligible for any platinum-based chemotherapy.1,2 Patients needed to have not received any prior systemic therapy for metastatic disease, have an ECOG performance statis of 0 to 2, and have no contraindications for immunotherapy. Other key eligibility criteria included being at least 18 years old, having measurable disease per RECIST 1.1 criteria, and having adequate hematologic and organ function.2
Eligible patients were randomly assigned 1:1 to receive 3 or 6 cycles of gemcitabine plus cisplatin/carboplatin.1 Patients in both arms also received maintenance therapy with avelumab for up to 2 years. Stratification occurred based on investigators’ choice of frontline chemotherapy (cisplatin vs carboplatin) and the presence of liver metastases (yes vs no).
The primary end points were changes in PROs per the EORTC QLQ-C30 global health status/QOL scale and overall survival (OS). Secondary end points included other PROs, safety and tolerability, progression-free survival (PFS), overall response rate, and best overall response.
At baseline, the median age in the overall population (n = 267) was 71 years (range, 44-91). Most patients were 65 years or older (73%), male (72%), did not have liver metastases (81%), had an ECOG performance status of 0 (73%), and received cisplatin plus gemcitabine (59%).
What were the additional efficacy and safety data?
Preliminary OS data showed that the median OS values were similar between the 3- and 6 cycle arms, at 18.92 (95% CI, 12.81-not reached [NR]) and 18.86 months (95% CI, 13.93-NR), respectively (HR, 1.15; 95% CI, 0.72-1.86; P = .56). Similarly, the median PFS was 8.0 months (95% CI, 6.70-11.89) vs 9.0 months (95% CI, 6.87-12.71), respectively (HR, 1.053; 95% CI, 0.725-1.527; P = .788).
Responses were also similar between the 2 arms. Response-evaluable patients who received 3 cycles of chemotherapy (n = 95) experienced a complete response (CR) rate of 13% and a partial response (PR) rate of 48%. The respective CR and PR rates in the 6-cycle arm (n = 100) were 13% and 46%.
In terms of safety, grade 1/2 treatment-related adverse effects (TRAEs) occurred at respective rates of 37% and 46% in the 3- and 6-cycle arms. Serious adverse effects (AEs; 35% vs 37%), grade 5 AEs (2% vs 0%), and discontinuation of chemotherapy due to TRAEs (2% vs 10%) were also reported. In the overall population (n = 1197), the most common any-grade TRAEs included anemia (9%), neutropenia (9%), nausea (8%), and fatigue (6%).
“We cannot claim noninferiority [in terms of OS] because of the trial design, [however] OS is still evolving. We will need more data with longer follow-up,” Grande said
Disclosures: Grande received honoraria for speaker engagements, advisory roles, or funding of continuous medical education from AbbVie, Adium, Advanced Accelerator Applications, Astellas, AstraZeneca, AVEO, Bayer, Bristol Myers Squibb, Clovis-Oncology, Dr. Reddy’s Eisai, Esteve, Eusa Pharma, GSK, IMVAX, IPSEN, ITM-Radiopharma, Janssen, Lilly, Merck KGaA, MSD, Novartis, Palex, Pfizer, Raffo, Roche, Rovi, and Tecnofarma. He received research grants from Astellas, AstraZeneca, IPSEN, Merck KGaA, Nanostring Technologies, Pfizer, and Roche. He has leadership roles in ENETS, ESMO, GETNE, Grupo Centro Tumores Genitourinarios, and GUARD consortium. He has stock or ownership interst in Amarin Corp, Bicycle Therapeutics, and Phamamar S.A.

Researchers at Rice University have engineered living cells to use a 21st amino acid that illuminates protein changes in real time, providing a new method for observing changes within cells. The technique is effective in bacteria, human…

Comorbid anxiety symptoms may diminish the
“The negative effects of comorbid anxiety may, in part, be explained by greater baseline depression…

Use of the Enhanced Recovery After Surgery (ERAS) protocol improved certain post-surgical outcomes in patients who had undergone elective gastric or colorectal cancer surgery, according to recently published data.
Özlem Kıvanç, MSc, RN, of…

As fall unfolds, Disney continues to captivate audiences with a slate of record-breaking premieres, fan-favorite series, and the thrill of live sports. This best-in-class storytelling and portfolio of brands, combined with Disney…

DURBAN, South Africa — A top executive at Africa’s biggest drug company shared a few home truths with the continent’s health policymakers about the obstacles to local manufacturing at the Conference on Public Health in Africa (CPHIA) 2025.
Aspen Pharmacare’s Dr Stavros Nicolaou blamed regulatory bottlenecks and procurement policies for the failure of drug manufacturers on the continent to realise their potential.
“It is unacceptable for African manufacturers to undergo a six-year [qualification] process before you get to market. We can do this in half the time,” he told a conference plenary session on local manufacturing on Thursday.
He is both Aspen’s group senior executive for strategic trade and chair of the industry body, the Pharmaceutical Manufacturers in South Africa.
It is a myth that African manufacturers are uncompetitive, added Nicolaou. For example, Aspen is active in 55 markets, reaches patients in over 150 countries, and is the global leading supplier of generic anaesthetics outside of the US.
Nicolaou called for a shift in multilateral procurement, including by Gavi, UNICEF and the Global Fund to Fight Aids, Tuberculosis and Malaria.
He told delegates that the establishment last year of the African Vaccine Manufacturing Accelerator (AVMA) was “a start”, but that the accelerator is “not fit for purpose” in its present form.
AVMA is a financing mechanism set up to raise $1.2 billion for manufacturers over 10 years. Nicolaou told Health Policy Watch that this amount – earmarked for “fill-and-finish” drug manufacturers – was rather modest.
He feels that there is insufficient incentive to spur the growth of the sector, upon which the future expansion of the medical products value chain depends.
“We can’t have African solutions compiled elsewhere and imposed on Africa. It won’t work.”
Pooled procurement of vaccines, therapeutics and diagnostics must be established “with speed”, he said.
Nicolaou noted that more than four years had passed and “nothing” had happened since the African Union and the Africa Centres for Disease Control and Prevention (Africa CDC) announced their ambition to ensure the continent manufactures 60% of its vaccine needs by 2040.
The AVMA launch came in the wake of the COVID-19 pandemic, which exposed the continent’s 99% reliance on foreign vaccine manufacturers and how its urgent needs were relegated to the back of the world procurement queue.
Nicolaou also responded to comments by South Africa’s Minister of Science, Technology and Innovation, Dr Blade Nzimande, who gave the session’s keynote address.
Nzimande called for efforts to “build sovereign capacity” in R&D, science and technology, including across the “whole health manufacturing value chain… be it therapeutic, diagnostic or vaccines we need for our continent”.
He described as “historic” the 60% by 2040 plan to develop tools to secure the continent’s health. He sketched how the initiative sought to expand capacity, implement health standards, and harmonise regulations — all themes elaborated on by other speakers and panellists at the session.
Nzimande toasted the initiative with a glass of water while at the lectern, encouraging his audience to join with applause.
“Government has an important role to play by acquiring locally produced therapeutics, diagnostics and vaccines,” he said.
Nicolaou said he was disappointed that Africa had the highest disease burden yet remained a serial importer and “every year the trade deficit in pharmaceuticals grows”.
South Africa and Egypt have the continent’s largest pharmaceutical markets.
“If you’re talking about security of supply for the continent, South Africa is immensely important. Charity starts at home in that we need to fix our own [national] procurement legislation first,” he said.
“Most of the volumes are procured via the state, and yet we continue to be a serial importer of pharmaceutical products in South Africa. The market is valued at R70-billion (manufacturers’ exit price)… and our trade deficit is more than 50% or around R39-billion.”
There was big potential for local production, yet South Africa continued to import high-volume products like antiretrovirals and vaccines, putting the brakes on local manufacturing development.
“There’s a heavy weighting towards importers, and we now have the data,” he said, citing customs figures, including information on imports from India.
“It demonstrates the extent of the problem. So we import significant and vast sums of our antiretrovirals. We have the largest HIV population of any country in the world; 17% of the world’s HIV population; there are about eight million infected people; about 6.6 million on treatment; and yet we continue to import most of our antiretrovirals.”
These imports were growing every year and, apart from antiretrovirals, included other high-volume products such as vaccines, TB medicines, and insulins.
He said this was despite local companies often being price competitive or representing “best value”: an opportunity to grow the local economy through the multiplier effect.
He proposed a three-point plan to remedy matters. First, the introduction of a priority review and parallel submission to expedite the licensing of medicines. The review of drugs by the national regulator should happen at the same time as the World Health Organisation’s review, instead of sequentially, which can add two to three years of costly delays.
Second, increase Gavi subsidies for the local production of vaccines to stimulate the market.
Third, establish a pool for procurement for the entire continent — as was successfully done during COVID-19 — to unlock economies of scale.
Also addressing the plenary, Nhlanhla Msomi, president of AfricaBio, called for a compact with the manufacturing industry to localise innovation.
However, Nicolaou said that it was premature to expect expansion of the value chain.
It was first necessary to support African manufacturers with fill-and-finish products to allow them to develop capacity and grow volume. In time, they could then invest in extending the value chain.
“Unless you start getting orders and you start succeeding in fill-and-finish first, companies are not going to backwardly integrate into drug [active] substance development,” he said.
Nicolaou said progress was not happening fast enough, and this was sapping momentum to achieve the “60% by 2040” aim.
“There’s a domestic issue to sort out, and then a continent,” he said.
Also at the plenary session, Dr Serge Blaise Emaleu, a global and public health and infectious diseases expert, said sustainable development could shift Africa from being an epicentre of disease to the centre of innovation.
Local manufacture was the “backbone of a sovereign health ecosystem”, but he cautioned that the commitment by African leaders to promote manufacture and invest in research “must be backed by financing” and that governance and leadership were required, and these things must be “moving in lockstep”.
Emaleu identified five interconnected pillars upon which Africa’s R&D self-reliance must be built: linking science to production; funding for research and development; investing in human resources; *building infrastructure and technology, and finally a regulatory framework to safeguard and sustain momentum.
Image Credits: Africa CDC, Rwanda Ministry of Health.
Combat the infodemic in health information and support health policy reporting from the global South. Our growing network of journalists in Africa, Asia, Geneva and New York connect the dots between regional realities and the big global debates, with evidence-based, open access news and analysis. To make a personal or organisational contribution click here on PayPal.

Aston Villa suffered a Europa League humbling as they were beaten 2-1 by Dutch minnows Go Ahead Eagles in Deventer.
Unai Emery’s side looked good to extend their winning streak to six matches in all competitions when they went ahead in the fourth…
Despite declining fertility in their midlife, many